Sterifre Medical Granted EPA Registration for Automated, Point of Care Disinfectant System to Help Combat COVID-19 and Other Healthcare Associated Pathogens
With the Omicron variant spreading exponentially and hospitals again being overwhelmed in the latest wave of the ongoing pandemic, Sterifre Medical, Inc. is thrilled to announce a milestone development in the fight against COVID-19 and other contagious pathogens in health care settings.
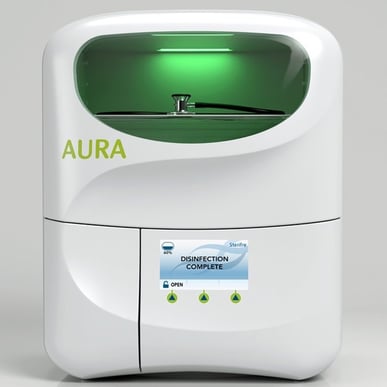 The U.S. Environmental Protection Agency (EPA) has granted Sterifre registration to begin commercial deployment of the company’s novel, automated device disinfectant system. The AURA™ system is believed to be the first registered, automated, point-of-care solution providing fast disinfection for non-critical medical devices and common articles used in the healthcare environment. It replaces the need for cumbersome and often challenging manual disinfection with wipes, reducing exposure of health care workers and patients alike to pathogens that cause healthcare associated infections (HAI).
The U.S. Environmental Protection Agency (EPA) has granted Sterifre registration to begin commercial deployment of the company’s novel, automated device disinfectant system. The AURA™ system is believed to be the first registered, automated, point-of-care solution providing fast disinfection for non-critical medical devices and common articles used in the healthcare environment. It replaces the need for cumbersome and often challenging manual disinfection with wipes, reducing exposure of health care workers and patients alike to pathogens that cause healthcare associated infections (HAI).
Research finds up to 97% of common healthcare equipment may be contaminated with multiple organisms. Solving the procedural challenges of manual disinfection has been the focus of Sterifre’s team of expert founders and industry leaders for a number of years. The EPA registration allows us to now bring the AURA system to market and help improve the wellbeing of healthcare workers and patients alike.
Utilizing a patented application of hydrogen peroxide and activated oxygen to inactivate microorganisms that cause disease, the AURA system not only helps reduce exposure to potentially infectious pathogens such as COVID-19, it significantly reduces the amount of time spent on manual disinfection. It’s also more cost effective than current methods, and can improve the quality of direct patient care. And the AURA disinfection system is compact and easy to use. It plugs in to a standard electrical outlet in any hospital or clinic patient care area, taking up about the same counterspace as a laptop computer.
“Our mission has always been to make healthcare safer,” says Mike Goonewardene, chief commercial officer at Sterifre. That mission took on even greater importance for us at our offices in Kirkland, Washington, as the city became the epicenter of the United States’ first COVID-19 outbreak in 2020. “Our first-of-its kind AURA system was designed to address many of the challenges that healthcare facilities face today. Sterifre is committed to putting fully validated, approved, state-of-the-art tools in the hands of healthcare workers,” he says.
As a regulator for microbicides used for hospital disinfection, the EPA’s registration for Sterifre recognizes the company conducted extensive microbial testing using EPA approved protocols at a laboratory under industry standard “Good Lab Practice (GLP)”. Sterifre also meets the EPA approval requirements under the Emerging Viral Pathogens Program for inactivating SARS-CoV-2, the coronavirus that causes COVID-19, because the AURA system has demonstrated effectiveness against viruses similar to SARS-CoV-2.
“The EPA is very focused on providing access to innovative solutions like our AURA disinfection system. Our approval and subsequent filings are for a broad set of efficacy claims,” explains Richard Shea, chief executive officer at Sterifre. "We are working closely with healthcare leaders across the country to deploy AURA as quickly as possible.”
Outmoded Approaches Don’t Meet Current Demands
Studies show up to 52% of health care workers are unaware of proper disinfection guidelines. Manual disinfection processes typically require the entire surface of an item remains wet with disinfectant for up to five minutes to thoroughly kill microorganisms. This timing may vary by organism and disinfectant product. And research finds items that have been improperly wiped may not be thoroughly disinfected, increasing the risk of pathogen transfer. With the AURA system, that’s no longer a problem.
Manual disinfection is not only time consuming and potentially ineffective, the harsh chemicals used can also impact caregiver health. According to a JAMA Network Open journal article, recurrent disinfectant chemical exposure is associated with a 25-38% increased rate of Chronic Obstructive Pulmonary Disease (COPD) in frontline caregivers.
And manual disinfection creates a significant environmental impact. It’s not widely known disinfectant wipes are made from unwoven, non-biodegradable plastic fiber, and U.S. hospitals discard almost 8 billion wipes annually.
“With increasing demands on our frontline healthcare workers, and concern for staff and patient safety, we’ve recognized for years an automated, hands-free solution is absolutely needed,” Shea explains. “Even under normal circumstances, an enormous amount of medical equipment is manually disinfected multiple times each day using billions of chemical wipes. That time and environmental impact is real.”
AURA™ Slows the Spread of Germs, Increases Pace of Patient Care
“AURA is the first automated disinfection process that can be used on a host of every day medical equipment such as thermometers, glucometers, otoscopes, stethoscopes, oximeters, pads/sensors, cords/cables, doppler probes and many more,” says Mark Golkowski, Ph.D, AURA™ co-inventor and Professor and Associate Dean in the College of Engineering, Design and Computing at the University of Colorado Denver.
“In replacing wipes and sprays, the system allows doctors, nurses and patients to finally do what we have known needs to be done to reduce exposure to infectious organisms but was not previously practical or in many cases possible,” emphasizes Golkowski, a Sterifre co-founder and member of Sterifre’s board of directors.

The AURA technology uses a well-recognized disinfectant that is consumed in the process, leaving no residual disinfectant. The patented application of hydrogen peroxide and activated oxygen creates an environment that efficiently kills bacteria and viruses including many multi-drug-resistant organisms, but without any damage to equipment.
The visionary AURA system is a testament to persistance, collaboration and innovation. It’s the brainchild of co-inventor Czeslaw Golkowski, Ph.D, a former Cornell University professor whose scientific and entrepreneurial vision and work led him to develop the groundbreaking disinfection technology and the early prototypes in partnership with his son Mark.
Even though his federal funding ran out, Golkowski persevered on his own, convinced of AURA’s potential to transform healthcare. After leaving the university, he ultimately became the first “non-Cornell” technology admitted to the prestigious McGovern Center for Venture Development in the Life Sciences incubator program at Cornell. Through the program, Golkowski continued to refine and prove his technology and the system, partnered with veteran leaders from the medical industry with notable track records of success, and would join them in forming Sterifre upon graduating from the program.
“We’re proud that the McGovern Center has had a hand in Sterifre’s development,” says Emmanuel Giannelis, vice president for research and innovation at Cornell. “Sterifre’s success demonstrates the strong entrepreneurial engine on our campus. Bringing the potential benefits of research innovations like the AURA system to a broad audience requires mentorship to develop a solid business foundation.”
AURA’s engineering and design is just as much a testament to innovation as its disinfection abilities. Sterifre partnered with nationally renowned design firms Nottingham Spirk and Simplexity Product Development to create a powerful, elegant, economical unit with a footprint no larger than a laptop. Since the only byproduct of AURA is clean air, it can be used anywhere there is an electrical outlet. The system continuously monitors treatment parameters and automatically indicates when disinfection is completed.
“Seeing the evolution from Nottingham Spirk’s initial work to the final product by our partners at Simplexity has been an incredible journey. We couldn’t be more thrilled with the result,” says Shea.
Simplexity CEO Dorota Shortell says she’s equally thrilled with AURA. “Even though we have developed hundreds of commercially viable products, we are especially excited about the Sterifre AURA system as it helps prevent the spread of infectious disease in an environmentally friendly manner,” she says. “It has been a great engineering challenge to apply decades of Simplexity’s expertise in systems engineering, fluidics, acoustics, injection molding part design, and electronics to help Sterifre bring such an innovative product to market.”
AURA disinfection systems are now available for pre-order with delivery beginning in 2022.
About Nottingham Spirk
Nottingham Spirk is a world-class product innovation firm with an unrivaled record of developing and commercializing disruptive consumer products, medical devices, digital IoT products and connected industrial products. We collaborate with Fortune 1,000 companies, middle market companies and funded venture companies to discover, design and execute product innovation programs and strategic business platforms that will delight customers, grow markets, and generate new revenue streams. Learn more about our innovation approach.
Submit a comment