Sterifre AURA™
Medical Devices
Fast point-of-care disinfectant system addresses infection control without the need for heat or harsh chemicals

Fast and flexible infection control
Hospital acquired infections (HAI) are the #1 quality and safety issue for healthcare facilities, affecting nearly 5% of all patients and causing 100,000 deaths annually. This is greater than the number of deaths from diabetes and prostate cancer combined.
These cases burden the U.S. healthcare industry with over $35B a year in unreimburseable costs, fueling sales of $1.5B worth of infection prevention products.
This legacy market has existing solutions, ranging from low cost disinfectant wipes and sprays to expensive capital equipment. However, none of these current technologies completely addresses the growing needs of this market and, in particular, the demand for fast and flexible point-of-care infection control.
Sterifre Medical Inc. (Sterifre) is commercializing a flexible disinfection process that is poised to address the growing infection control challenges of healthcare facilities. The company’s patented AURA System is comprised of microspray hydrogen and cold plasma ozone in unique combinations, operating at room temperature and atmospheric pressure.
These conditions enable fast point-of-care disinfection of a broad range of devices and sensitive materials commonly used in healthcare facilities, including plastics, metals, glass and electronics. This contrasts with existing technologies, which employ harsh chemicals, heat, and/or operate under vacuum, ultimately limiting material and device compatibility. Additionally, the system is designed to reduce ongoing capital and operating expenses for the customer.
The company's been granted expedited registration with the Environmental Protection Agency, which regulates microbicides used for hospital disinfection. Sterifre is concurrently seeking EPA approval under the Emerging Viral Pathogens Program for inactivating SARS-CoV-2, the coronavirus that causes COVID-19.
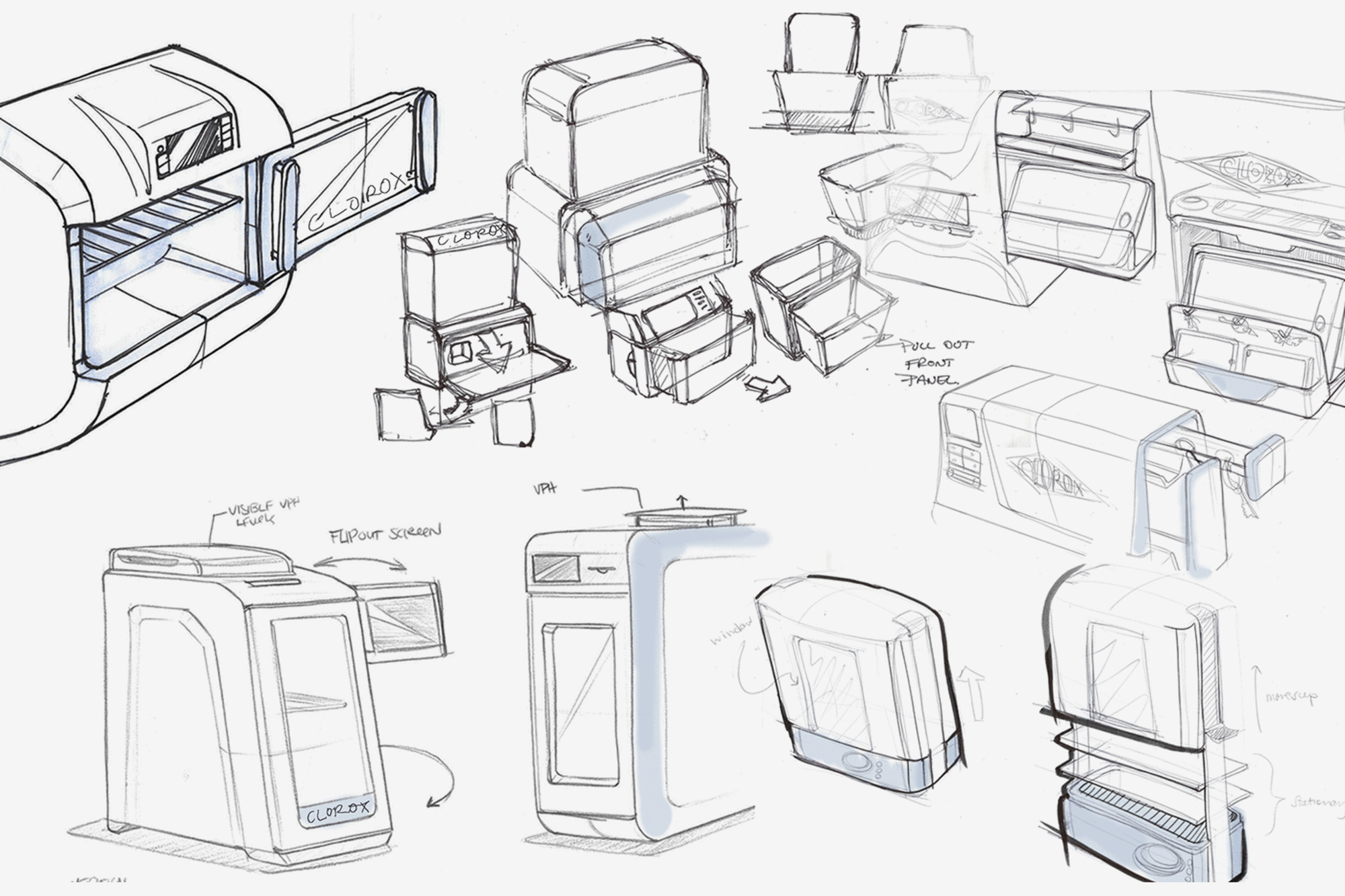
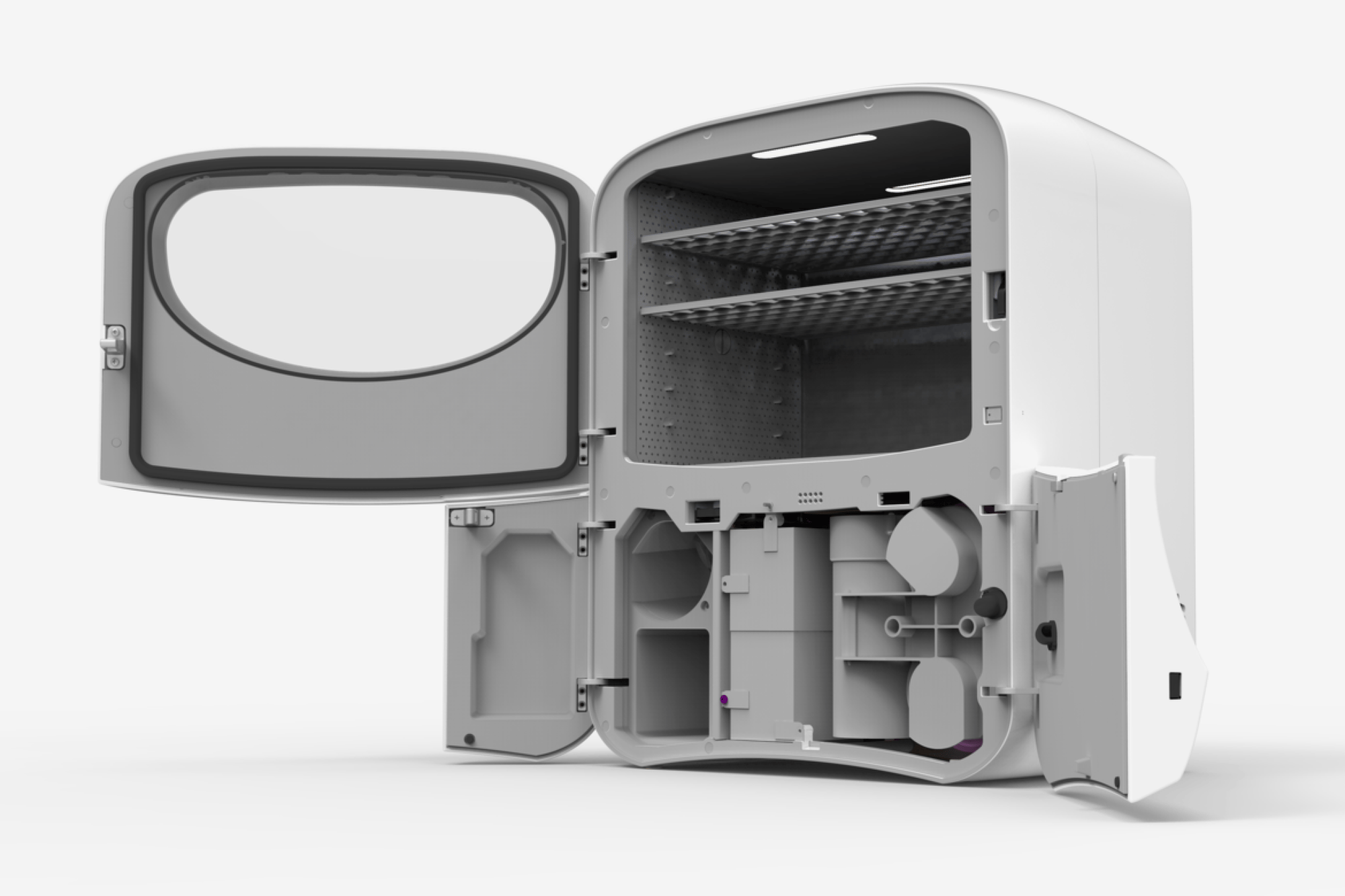
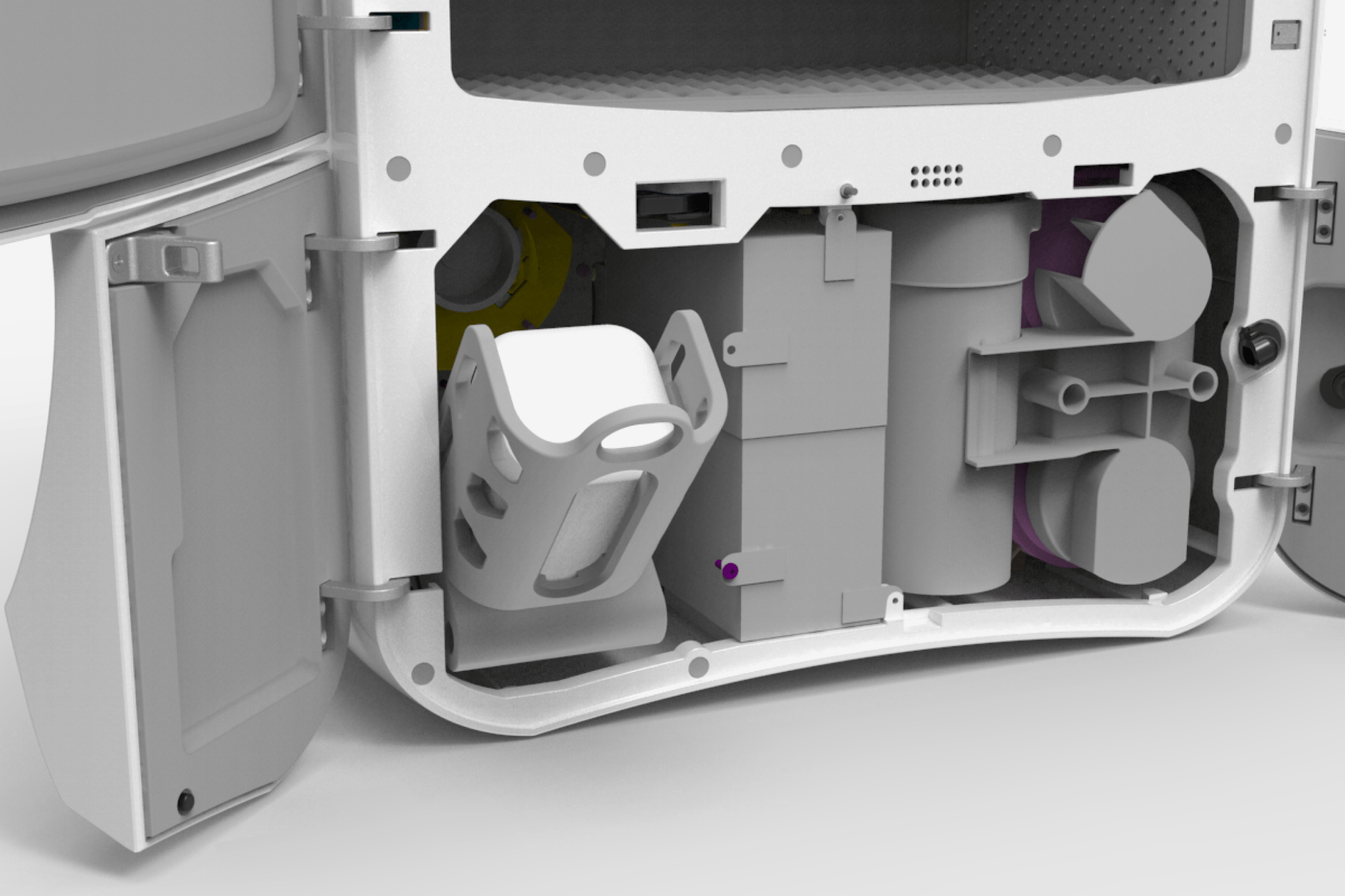
The Aura competitive advantage
AURA is an elegant technology that manifests itself in a relatively simple design. The system was designed with low cost of goods and “plug and play” features that require little personnel training.
AURA can be configured to process items at point-of-care, including both countertop & wall-mounted units and large volume industrial units. This enables Sterifre to develop products specific to application, market segment, and environment of use. Such scalability is derived from the initial product designs, which were microwave oven-sized, weighing 23 lbs.
The design requires no special electrical, plumbing, or ventilation connections, a feature carried forward in the company’s first product. This design enables point of care applications, outside of central sterile processing, where existing infection control products (wipes, sprays, hand sanitizers) do not fully address healthcare facility compliance and material compatibility needs.
Ultimately, AURA will enable hospitals to:
- Ensure compliance with infection prevention protocols
- Automate existing manual processes to allow more hands-on patient care
- Reduce the environmental impact of discarding chemically treated wipes
- Reduce the incidence of equipment damage from harsh chemicals during disinfection with wipes
- Protect staff from health risks associated with occupational exposure to disinfectants
Our Work
Medical Devices
Cardinal Health InPower™
A connected consumer medical device to improve medication adherence rates.



